"During the third quarter of fiscal 2018, InMed continued to execute on its R&D and business strategy," stated President and Chief Executive Officer, Eric A. Adams. "InMed is making headway at establishing its leadership position as a fully-integrated cannabinoid pharmaceutical company; we are not only developing innovative therapies for the treatment of important diseases with high unmet medical needs, but are also leading the burgeoning cannabinoid sector with our first-in-class biosynthetic manufacturing technology. "Over the next several months," Mr. Adams concluded, "we will remain focused on building shareholder value via innovative scientific research and development, as well as attaining greater exposure within the investment community in the United States."
Business and R&D Update:
Biosynthesis manufacturing technology. The Company, in conjunction with its collaborators at the University of British Columbia, continued to advance the production platform for the bio-fermentation of cannabinoids. Optimization of the vector will continue in parallel with the identification of optimal fermentation conditions and downstream purification processes with yet-to-be announced 3rd party suppliers, who are currently being vetted.
INM-750 for the treatment of the rare disease epidermolysis bullosa (EB). During the three months ended March 31, 2018, the Company continued working to finalize the formulation for INM-750 and initiated work on IND-enabling pharmacology and toxicology studies. The Company currently anticipates that it will be discussing its clinical development plans with regulatory authorities in the second half of 2018. After further data analysis in preparation for these discussions, InMed has determined that additional pre-clinical pharmacology experiments are warranted. We now expect to file our Investigational New Drug ("IND") application in the second half of calendar-year 2019. These experiments will be designed to ensure that INM-750 meets the clinical needs of EB patients and improve the overall prospects of success for the INM-750 development program. In particular, these experiments will help us better understand the contributions of each cannabinoid component as well as their final combination in INM-750. Specifically, these additional pre-clinical experiments will focus on the effects these compounds have on wound healing and the reduction of inflammation.
INM-085 for the treatment of glaucoma. InMed announced the publication of a peer-reviewed article in Drug Delivery and Translational Research on March 6, 2018. The article, titled "A stimulus-responsive, in situ forming, nanoparticle-laden hydrogel for ocular drug delivery", presents results from a pre-clinical study co-sponsored by InMed and was co-authored by Dr. Sazzad Hossain, InMed's Chief Scientific Officer. Data from this study support what the Company believes to be a first-in-class nanoparticle-hydrogel formulation for cannabinoid delivery to the eye, resulting in significant delivery of cannabinoids to the eye via transcorneal penetration, resulting in a 300% increase versus a control formulation. Next steps in this program will include additional preclinical efficacy testing and advanced formulation development.
On March 13, 2018, InMed announced the appointment of Eric Hsu, Ph.D., as Vice President of Preclinical Research and Development.
Corporate Update (expressed in Canadian Dollars):
During the quarter ending March 31, 2018, the Company issued an aggregate 25,143,531 common shares, pursuant to InMed's $9.4 million private placement (13,428,571 shares issued) completed on January 3, 2018, as well as the exercise of investor and agents' warrants (5,648,947 shares issued), and stock options (6,030,295 shares issued) and as consideration for services provided (35,718 shares). At March 31, 2018, the Company's total issued and outstanding shares were 152,792,997. During 3Q18, the weighted average number of common shares was 151,537,571, which is used for the calculation of loss per share.
Effective March 26, 2018, the Company commenced trading on the Toronto Stock Exchange.
Results of Operations (expressed in Canadian Dollars):
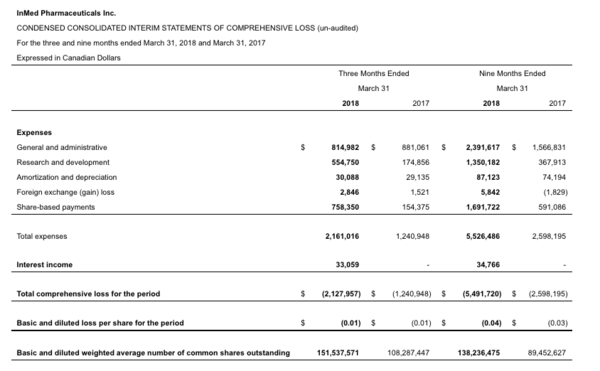
For the three and nine months ended March 31, 2018, the Company recorded a net loss of $2.13 million and $5.49 million or $0.01 and $0.04 per share, respectively, compared with a net loss of $1.24 million and $2.6 million, or $0.01 and $0.03 per share, for the three and nine months ended March 31, 2017.
Research and development expenses were $0.55 million for the three months ended March 31, 2018, compared with $0.17 million for the three months ended March 31, 2017. For the nine months ended March 31, 2018, research and development expenses totaled $1.35 million, which compares with $0.37 million for the corresponding period in 2017. The increase in research and development expenses in the three months ended March 31, 2018 as compared to the same quarter in 2017 was primarily due to increased spending with external contractors for expenditures related to the advancement of INM-750 as a treatment for EB.
The Company incurred general and administrative expenses of $0.81 million for the three months ended March 31, 2018, compared with $0.88 million for the three months ended March 31, 2017. For the nine months ended March 31, 2018, general and administrative expenses totaled $2.39 million, which compares with $1.57 million for the corresponding period in 2017.
The Company also incurred non-cash, share-based payments, in connection with the grant of stock options, of $0.76 million for the three months ended March 31, 2018, compared with $0.15 million for the three months ended March 31, 2017. For the nine months ended March 31, 2018, non-cash, share-based payments totaled $1.69 million, which compares with $0.59 million for the corresponding period in 2017.
At March 31, 2018, the Company's cash, cash equivalents and short-term investments were $13.88 million, which compares to $6.71 million at June 30, 2017. During the nine months to March 31, 2018, the Company's cash, cash equivalents and short-term investments increased by $7.17 million, which primarily resulted from net cash proceeds of $8.63 million from with its non-brokered private placement completed on January 3, 2018 and $1.90 million proceeds from the exercise of warrants and stock options less $3.30 million cash outflows from operating activities.
Table 1: Condensed consolidated interim statements of financial position (un-audited):
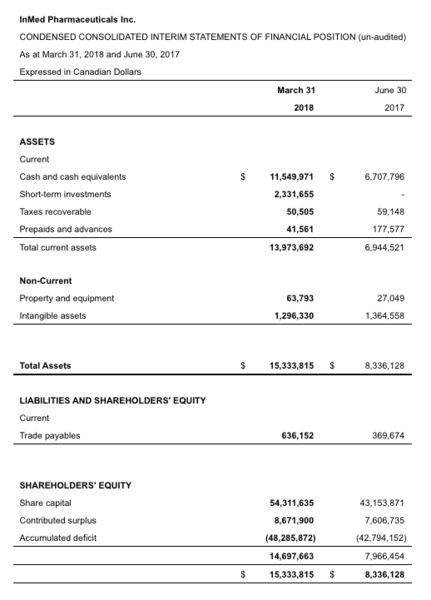
Table 2: Condensed consolidated interim statements of comprehensive loss (un-audited):
The Company's full financial statements and related MD&A for the three and nine months ended March 31, 2018 are available at www.sedar.com.
About InMed:
InMed is a pre-clinical stage biopharmaceutical company that specializes in developing novel therapies through the research and development into the extensive pharmacology of cannabinoids coupled with innovative drug delivery systems. InMed's proprietary bioinformatics database drug/disease targeting tool, cannabinoid biosynthesis technology and drug development pipeline are the fundamental value drivers of the Company. For more information, visit www.inmedpharma.com
About Epidermolysis Bullosa (EB).
EB is a group of rare diseases that cause fragile, blistering skin. The blisters may appear in response to minor injury, even from heat, rubbing, scratching or adhesive tape. In severe cases, the blisters may occur inside the body, such as the lining of the mouth or the stomach. Most types of epidermolysis bullosa are inherited. The condition usually presents in infancy or early childhood. Epidermolysis bullosa has no cure.
About INM-750.
INM-750 is a proprietary, topical cannabinoid product candidate targeted as a therapy in epidermolysis bullosa (EB) and other potential dermatological and wound-healing applications. It has been specifically designed with the intent to: (i) modify the underlying cause of the disease in certain patients with EB Simplex (EBS, the most common form of EB), and (ii) to treat the major symptoms of the disease in all patients with EB. Preclinical data generated previously demonstrates that INM-750 may have a significant impact on certain symptoms of EB (which may include improvement of wound area to promote healing, reduction in pain, itch and inflammation, and providing antimicrobial activity). These disease hallmarks are key therapeutic targets for the effective treatment of EB as well as several other dermatological conditions. Additionally, our data indicate that INM-750 may have an impact on the underlying disease by increasing the production of certain proteins, called keratins, in the skin.
About Glaucoma.
Glaucoma is a group of eye conditions that damage the optic nerve, which is vital to good vision. This damage is often caused by an abnormally high pressure in your eye. It is the second leading cause of blindness worldwide. It can occur at any age but is more common in older adults. The most common form of glaucoma has no warning signs. The effect is so gradual that you may not notice a change in vision until the condition is at an advanced stage. Vision loss due to glaucoma can't be recovered.
About INM-085.
InMed is developing a stimulus-responsive, nanoparticle-laden vehicle for controlled delivery of ophthalmic drugs into the aqueous humor of the eye. The first application of this delivery vehicle will be for INM-085 as a cannabinoid-based topical therapy to reduce the intraocular pressure associated with glaucoma as well as to serve as a neuroprotectant to the retinal ganglion cells. INM-085 is intended for application as a once-per-day eye drop administered immediately prior to the patient's bedtime, intending to assist in reducing the high rate of non-adherence with current glaucoma therapies. Additionally, this novel, proprietary delivery system for ocular drugs may also play an important role in enabling other companies' proprietary ocular drug candidates or re-invigorating the commercial potential of off-patent products that would benefit from a once-a-day dosing regimen.
Read More


